Is Enthalpy A State Function
This is the change in internal energy that is equal to the heat transfer. Enthalpy h is a state function because it is defined solely in terms of other state functions.

State Function Explanation Youtube
1 H U P V.
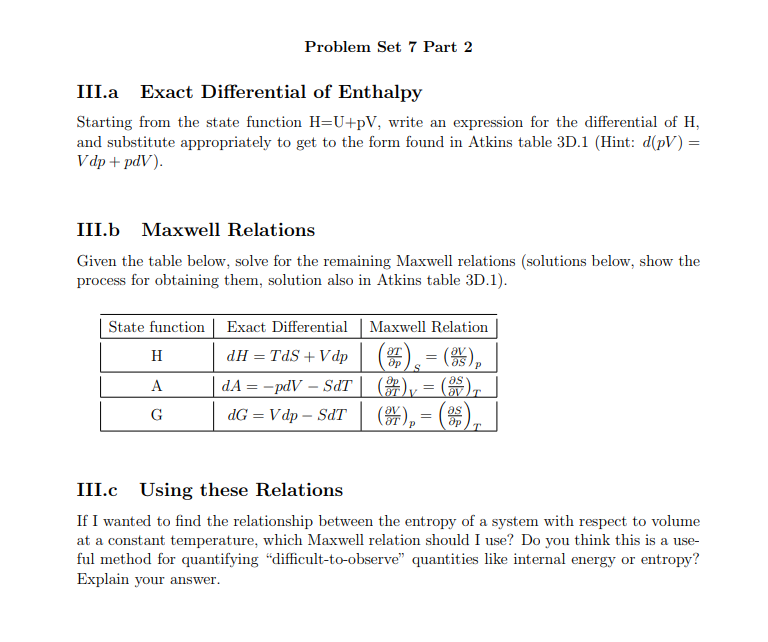
. Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that occurred for it to reach. U P and V are all state functions. H U P V.
Enthalpy is an energy-like property or state functionit has the. This means that enthalpy depends only on the final energy pressure and volume and not the path the system took to get to the final state. Density ρ The above is the list of properties that depends on the state.
Since enthalpy is a state function and change in enthalpy can be. Answer 1 of 2. More specifically state functions facilitate the use of Hesss Law which allows the manipulation addition subtraction multiply etc of the enthalpies of half reactions when adding multiple half reactions to form a full reaction.
What is a state function Class 11. H U P V. The volume of water in a pond can increase naturally by rainwater or by artificially.
Their values depend only on the state of the. Answer 1 of 5. Hesss Law is dependent upon the fact that enthalpy is a state function.
Are work and heat state functions. When a process occurs at constant. The state functions are also known as state variables in thermodynamics.
Enthalpy is a state function because it depends only on two thermodynamic properties of the state the substance is at the moment like temperature and pressure or. If you change volume from say 1 L to 10 L. It is a state function at constant pressure used in chemical and biological systems.
Enthalpy the sum of the internal energy and the product of the pressure and volume of a thermodynamic system. Internal energy u 4. Hi Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that.
The enthalpy change Δ H H2 H1 will thus be independent of the path used to travel from state 1 to state 2. Enthalpy H has to do with thermodynamics. Work and heat state functions are the.
The expression for enthalpy can be written as follows. In the c See more. Difference Between State Function And Path Function.
As a state function enthalpy depends only on the final configuration of internal energy pressure and volume not on the path taken to achieve it. This is a new state function. How does enthalpy change.
A state function Class 11 is a function that returns a boolean value. This corollary is of course the basis of Hess law. The value is noted for initial stage and the final stage.
Enthalpy Entropy Free Energy Internal Energy. Where u p and v are the specific internal energy the pressure and the specific. Enthalpy is a state function because it is defined in terms of state functions.
Enthalpy H is the sum of the internal energy U and the product of pressure and volume P V given by the equation. As seen in the above example enthalpy is a state function because its value depends only on initial and final conditions. Enthalpy is defined as H UPV The reason that H is a state function is that all three functions V P and U are state functions too.
Q P Δ U P Δ V U 2 U 1 P V 2 V 1 q P U 2 P V 2 U 1 P V 1 We can define this term as enthalpy. Therefore enthalpy is a state function.
Which One Is Not A State Function
State Versus Path Functions
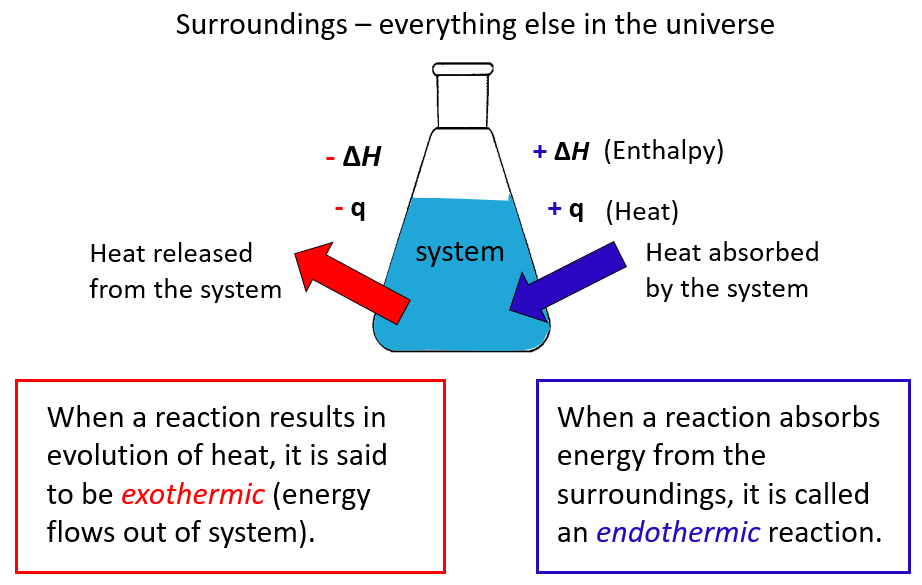
What Is Enthalpy Chemistry Steps

Answered Which One Of The Following Is Not A Bartleby

State Functions In Thermochemistry Overview Examples Video Lesson Transcript Study Com

What Is Enthalpy In Thermodynamics Understanding Business Analysis And Engineering Principles

Slides On Enthalpy And Its Function Slides Chemistry Docsity

Enthalpy And Volume Of Different State Of Drugs As A Function Of Download Scientific Diagram

State Function Definition Equation And Example

Solved Which One Of The Following Thermodynamic Quantities Is Not A State Function Entropy Enthalpy Work Gibbs Free Energy
Enthalpy Vs Entropy Thermodynamics Psiberg

Enthalpy As A State Function Youtube

State Functions In Thermochemistry Overview Examples Video Lesson Transcript Study Com

Enthalpy Really Is A State Function Hess S Law Chem 152 Study Notes Chemistry Docsity

Difference Between State Function And Path Function Compare The Difference Between Similar Terms
Chem 245 Enthalpy

State Functions Thermodyanmics Concepts Explanation Embibe